1) Discovering various ways to separate solids
Technique #1: Water
"The water made the moss float because it is lighter" (Student from 16A)
*Water was not as effective if materials are the same density
 Technique #2: Sieves
Technique #2: Sieves
"They were good because if you mixed everything together when you sieve it, the smaller ones go to the bottom and the larger ones go to the top". (Student from 16A)
"If you mix two things that are practically the same size it won't work because they won't seperate" (Student from 16B)
"The magnets worked for the nails because they are magnetic but the other materials weren't (like the glass) so they were stuck together" (Student from Room 16A)
 Experiment #2: Mixing Liquids
Experiment #2: Mixing Liquids
How did you know if something separated, partially separated or did not separate?
-"If you took the eye dropper and you had one that was heavier then the other you could have separated it from that" (Student Room A)
- "If you put two liquids and one is already separated with it and you mix it, and some of it is gone" Student on Room B (on how you know its partially separated)
Check out this great video that reviews vocabulary such as:
homogenous
heterogenous
dissolve
mixture
solution
Reversible and Irreversible Changes
Evaporation - Solids can be recovered by evaporating the water. The water is lost into the air as it evaporates while the solid remains behind.
Decanting - to pour off a liquid to leave behind a sediment
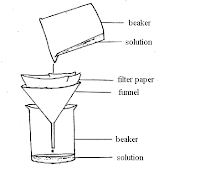
Filtering - Solids can be stirred up to form a suspension which can then be poured into a filtering system. Water will pass through the filter but the insoluble substance
Creating Crystals:
Crystals are a solid unit in which molecules have been arranged in repeating patterns or networks and are created by the process of evaporation
Students in Room 16 created their own crystals with the following procedure:
2. Tie a piece of string to the top of your decoration
3. Pour boiling water into your jar
4. Add 4 tablespoons of borax, continue until the water is cloudy. Once it is cloudy and no more will dissolve you're reached saturation.
5. Wrap the string horizontally onto your stick. Dip your decoration until completely under water.
Carbon Dioxide
Carbon dioxide can be produced through the interaction of solids and liquids. It is odorless, tasteless, colorless, heavier than air and does not burn. We learned about two different ways that carbon dioxide can be produce in chemistry.
Balloon Blow-up
Making Your Own Lava Lamp
Chemical Reactions
A chemical reaction has occurred when a substance changes color, odor, temperature (heat) or produces a gas. In a chemical reaction a new substance is created. Chemical changes are generally irreversible
An acid is a substance that can donate hydrogen ions.
Characteristics:
-Taste sour
-corrosive to materials
-can sting when touched
-become less acidic when mixed with bases
A base is a substance that can accept hydrogen ions.
Characteristics:
-Have a bitter taste
-Do not react when combined with most metals
-become less basic when mixed with acids



No comments:
Post a Comment